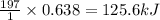Consider the combination reaction between sulfur dioxide gas and oxygen gas to produce sulfur trioxide. Also consider that you have 40.86 g of sulfur dioxide gas and 40.01 g of oxygen gas. For this reaction, use LaTeX: \Delta Δ Hrxn = - 197 kJ/mol of product. If the reaction proceeds to completion, what is the maximum amount of heat (in kJ) that you could expect to generate?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 13:30, christabell0303
Consider this reaction taking place in a closed 2 liter container: 2so2(g) + o2(g) → 2so3(g) if the volume of the container is decreased to 1 liter, what will happen to the equilibrium of the reaction? it will shift left. it will shift right. it will remain constant it will decrease by half
Answers: 3

Chemistry, 23.06.2019 17:00, Miloflippin9766
Which of the following has potential energy but no kinetic energy? longitudinal sound waves an arrow shot from a bow a compressed spring a vibrating atom
Answers: 1


Chemistry, 23.06.2019 19:30, kodyharris117
The following data was collected when a reaction was performed experimentally in the laboratory ; determine the maximum amount of alcl3 that was produced during the experiment explain how you determined this reactants products al(no3)3 nacl. nano3 alcl3 4 moles 9 moles ? ?
Answers: 3
Do you know the correct answer?
Consider the combination reaction between sulfur dioxide gas and oxygen gas to produce sulfur trioxi...
Questions in other subjects:




History, 26.02.2020 19:04


Chemistry, 26.02.2020 19:04





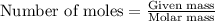 .....(1)
.....(1)
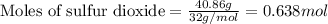
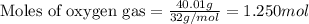
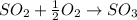
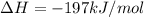
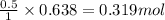 of oxygen gas
of oxygen gas