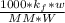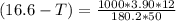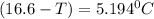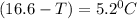Chemistry, 28.02.2020 19:15, noobgirlaskthequest
What is the freezing point of a solution that contains 12.0 g of glucose (C6H12O6) in 50 g of acetic acid (CH3COOH). For acetic acid, Kf is 3.90°C/m and the melting point is 16.6 °C

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 23.06.2019 01:30, ayoismeisalex
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 05:30, xarianna2007
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1

Chemistry, 23.06.2019 06:30, tdahna0403
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
Do you know the correct answer?
What is the freezing point of a solution that contains 12.0 g of glucose (C6H12O6) in 50 g of acetic...
Questions in other subjects:

Mathematics, 11.07.2019 01:40


Chemistry, 11.07.2019 01:40




Biology, 11.07.2019 01:40

History, 11.07.2019 01:40

Geography, 11.07.2019 01:40

Biology, 11.07.2019 01:40