
Chemistry, 28.02.2020 04:02, TrapQueen665
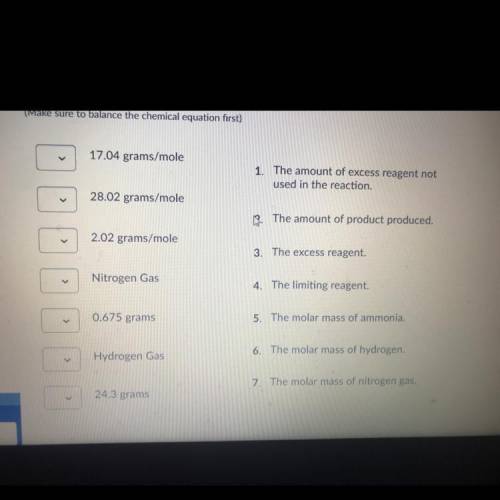
The haber process involves nitrogen gas combining with hydrogen gas to produce ammonia. If 20.0 grams of nitrogen gas combines with 5.0 grams of hydrogen gas, find the following: the molar mass of reactants and products, limiting reactant, the excess reactant, the amount of ammonia produced, the amount of excess chemical not used in the reaction.
Nitrogen gas+ hydrogen gas <——> ammonia gas
N2 + H2 -> NH3
(Make sure to balance the chemical equation first)
A lot of points !!!

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, asims13
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2


Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 22:20, trockout4868
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
Do you know the correct answer?
The haber process involves nitrogen gas combining with hydrogen gas to produce ammonia. If 20.0 gram...
Questions in other subjects:

Mathematics, 10.02.2021 19:20

Mathematics, 10.02.2021 19:20

Mathematics, 10.02.2021 19:20


Mathematics, 10.02.2021 19:20

Mathematics, 10.02.2021 19:20

Mathematics, 10.02.2021 19:20


English, 10.02.2021 19:20






