
Chemistry, 28.02.2020 02:23, melissapulido198
Consider the equilibrium between SbCl5, SbCl3 and Cl2. SbCl5(g) -->SbCl3(g) + Cl2(g) K = 2.30×10-2 at 566 K .The reaction is allowed to reach equilibrium in a 7.40-L flask. At equilibrium, [SbCl5] = 0.333 M, [SbCl3] = 8.75×10-2 M and [Cl2] = 8.75×10-2 M.
(a) The equilibrium mixture is transferred to a 14.8-L flask. In which direction will the reaction proceed to reach equilibrium?
(b) Calculate the new equilibrium concentrations that result when the equilibrium mixture is transferred to a 14.8-L flask.
[SbCl5] = M
[SbCl3] = M
[Cl2] = M

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
Do you know the correct answer?
Consider the equilibrium between SbCl5, SbCl3 and Cl2. SbCl5(g) -->SbCl3(g) + Cl2(g) K = 2.30×10-...
Questions in other subjects:



Chemistry, 24.10.2020 09:20


History, 24.10.2020 09:20


English, 24.10.2020 09:30

History, 24.10.2020 09:30


Social Studies, 24.10.2020 09:30


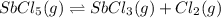
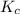
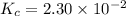
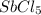 in 7.40 L = 0.333 M
in 7.40 L = 0.333 M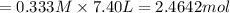
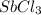 in 7.40 L =
in 7.40 L = 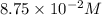
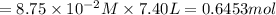
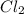 in 7.40 L =
in 7.40 L = 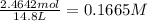
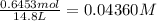
![K_c=\frac{[SbCl_3][Cl_2]}{[SbCl_5]}](/tpl/images/0527/6048/c5c78.png)
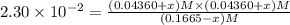
![[SbCl_5]=(0.1665-x) M=(0.1665-0.01536) M=0.1511 M](/tpl/images/0527/6048/35e3f.png)
![[SbCl_3]=(0.04360+x) M=(0.04360+0.01536) M=0.05896 M](/tpl/images/0527/6048/a8c15.png)
![[Cl_2]=(0.04360+x) M=(0.04360+0.01536) M=0.05896 M](/tpl/images/0527/6048/dab14.png)




