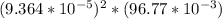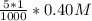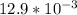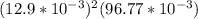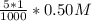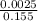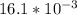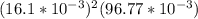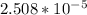For which of the following mixtures will Ag2SO4(s) precipitate?
a. 150.0 mL of 0.10 M Na2SO4(aq) and 5.0 mL of 0.20 M AgNO3(aq)
b. 150.0 mL of 0.10 M Na2SO4(aq) and 5.0 mL of 0.30 M AgNO3(aq)
c. 150.0 mL of 0.10 M Na2SO4(aq) and 5.0 mL of 0.40 M AgNO3(aq)
d. 150.0 mL of 0.10 M Na2SO4(aq) and 5.0 mL of 0.50 M AgNO3(aq)

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
Do you know the correct answer?
For which of the following mixtures will Ag2SO4(s) precipitate?
a. 150.0 mL of 0.10 M Na2SO4(a...
a. 150.0 mL of 0.10 M Na2SO4(a...
Questions in other subjects:








Chemistry, 19.08.2019 13:10

History, 19.08.2019 13:10

Mathematics, 19.08.2019 13:10


 =
= ![[Ag^+]^2[SO_4^{2-}]](/tpl/images/0527/4149/191c0.png)
 <
<  ; precipitation will not occur
; precipitation will not occur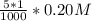
 =
= 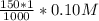
![[Ag^+]^2](/tpl/images/0527/4149/1aebd.png) =
= 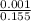

![[SO_4^{2-}]](/tpl/images/0527/4149/43b69.png) =
= 

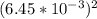 ×
×