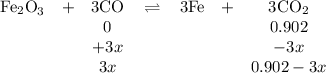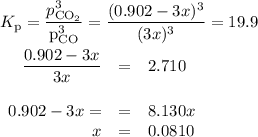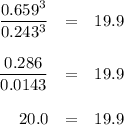Chemistry, 27.02.2020 22:41, bartekpiglo
At 1000 K, Kp=19.9 for the reaction Fe2O3(s)+3CO(g)<--->2Fe(s)+3C O2(g) What are the equilibrium partial pressures of CO and if CO2 is the only gas present initially, at a partial pressure of 0.902 ?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
Do you know the correct answer?
At 1000 K, Kp=19.9 for the reaction Fe2O3(s)+3CO(g)<--->2Fe(s)+3C O2(g) What are the equilibri...
Questions in other subjects:

English, 22.04.2021 23:20

Mathematics, 22.04.2021 23:20

History, 22.04.2021 23:20

English, 22.04.2021 23:20


Computers and Technology, 22.04.2021 23:20




History, 22.04.2021 23:20