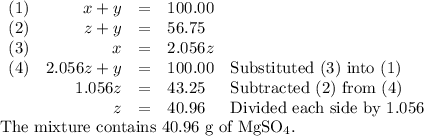
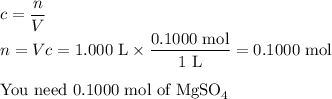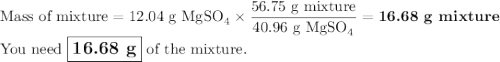Your lab partner accidentally mixed some sodium chloride with your sample of Epsom salts (MgSO₄・7H₂O). You want to make a standard solution of magnesium ion, and this is the only sample of a magnesium salt you have. To determine the amount of magnesium salt in the mixture, you heat 100.00 g to drive off the water of hydration and find that the anhydrous mixture has a mass of 56.75 g. How many grams of the original salt mixture must you add to 1.000 L of water to make a 0.100 M solution of Mg²⁺ ion? g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 18:00, brisacruz013
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 23.06.2019 14:20, bjbass899
Compounds a and b react to form compounds c and d according to the equation: aa + bb → cc + dd. under which conditions will the rate law be given by the equation: rate = k[a]a[b]b? a. the reaction takes place in one step. b. the reaction is endothermic. c. the reaction is exothermic. d. the reaction involves more than one step.
Answers: 3
Do you know the correct answer?
Your lab partner accidentally mixed some sodium chloride with your sample of Epsom salts (MgSO₄・7H₂O...
Questions in other subjects:



Health, 14.12.2021 06:20

Mathematics, 14.12.2021 06:20

Chemistry, 14.12.2021 06:20

Mathematics, 14.12.2021 06:20



English, 14.12.2021 06:20

Mathematics, 14.12.2021 06:20