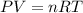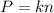Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g of Ne, and the other flask contains 10.0 g of He. Is each of the following statements true or false? Explain.1) True. Because the gases have the same mass, they exert the same pressures.2) True. Both the volumes and the temperatures of the gases are the same, which means that their pressures must be equal.3) False. There are different numbers of moles in the flasks, meaning that the pressures are different.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Do you know the correct answer?
Two flasks of equal volume and at the same temperature contain different gases. One flask contains 1...
Questions in other subjects:



Mathematics, 13.02.2021 06:00



Biology, 13.02.2021 06:00


Mathematics, 13.02.2021 06:00