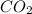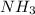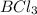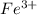Chemistry, 27.02.2020 20:17, airelle146
Identify from the following list of molecules and ions which behave as Lewis acids: CO2, NH3, BCl3, Fe3+. (A) CO2 and NH3 (B) NH3 and BCl3 (C) CO2 and Fe3+ (D) CO2, BCl3, and Fe3+ (E) CO2, NH3, BCl3, and Fe3+

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 23.06.2019 03:50, lindseyklewis1p56uvi
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2

Chemistry, 23.06.2019 04:30, Har13526574
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 13:20, brainbean
Use the periodic table to answer the following questions. what is the predicted order of first ionization energies from highest to lowest for beryllium, calcium, magnesium, and strontium? o be > ca > mg > sr o be > mg > ca > sr o ca > sr> be > mg o sr > ca > mg > be done
Answers: 1
Do you know the correct answer?
Identify from the following list of molecules and ions which behave as Lewis acids: CO2, NH3, BCl3,...
Questions in other subjects:


Mathematics, 17.10.2019 16:10