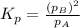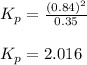Chemistry, 27.02.2020 19:50, superfly903
Consider the hypothetical reaction A(g)←→2B(g). A flask is charged with 0.77 atm of pure A, after which it is allowed to reach equilibrium at 0 ∘C. At equilibrium the partial pressure of A is 0.35 atm .A: What is the total pressure in the flask at equilibrium?
B:What is the value of Kp?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
Do you know the correct answer?
Consider the hypothetical reaction A(g)←→2B(g). A flask is charged with 0.77 atm of pure A, after wh...
Questions in other subjects:

Mathematics, 21.09.2019 11:10

Social Studies, 21.09.2019 11:10



Social Studies, 21.09.2019 11:10


Mathematics, 21.09.2019 11:10

Arts, 21.09.2019 11:10

Computers and Technology, 21.09.2019 11:10


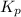 for the given equation is 2.016
for the given equation is 2.016
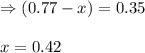
![p^A_{eq}+p^b_{eq}=[0.35+0.84]atm=1.19atm](/tpl/images/0527/0172/9c43a.png)