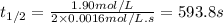The half-life of a reaction, t1/2, is the time required for one-half of a reactant to be consumed. It is the time during which the amount of reactant or its concentration decreases to one-half of its initial value.
Determine the half-life for the reaction in Part B using the integrated rate law, given that the initial concentration is 1.90mol?L?1 and the rate constant is 0.0016mol?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, lizethdominguez037
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
Do you know the correct answer?
The half-life of a reaction, t1/2, is the time required for one-half of a reactant to be consumed. I...
Questions in other subjects:

Chemistry, 21.08.2019 08:30

Mathematics, 21.08.2019 08:30




English, 21.08.2019 08:30





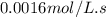
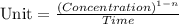
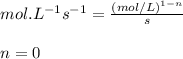
![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0526/7626/b5b11.png)
![[A_o]](/tpl/images/0526/7626/dc622.png) = initial concentration = 1.90 mol/L
= initial concentration = 1.90 mol/L