
Chemistry, 27.02.2020 05:24, arianaguerin
What mass of NH3 (in grams) must be used to produce 5.65 tons of HNO3 by the Ostwald process, assuming an 80.0 percent yield in each step (1 ton = 2000 lb; 1 lb = 453.6 g)? Enter your answer in scientific notation.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, goodygoodgirlygirl
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1


Chemistry, 23.06.2019 07:00, asims13
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
Do you know the correct answer?
What mass of NH3 (in grams) must be used to produce 5.65 tons of HNO3 by the Ostwald process, assumi...
Questions in other subjects:

Mathematics, 10.08.2021 03:10


Mathematics, 10.08.2021 03:10

Mathematics, 10.08.2021 03:10


History, 10.08.2021 03:10


Mathematics, 10.08.2021 03:10



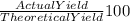 = 80 %
= 80 %



