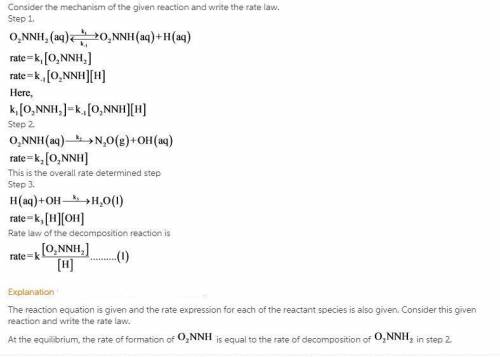
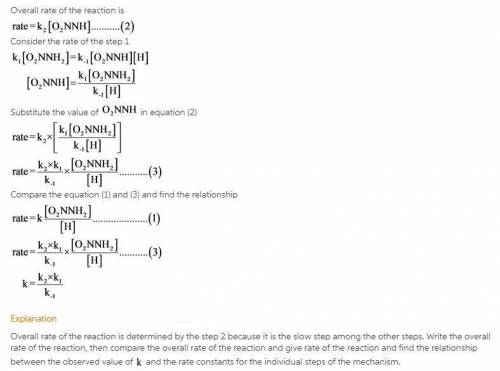
The decomposition of nitramide, O 2 NNH 2 , in water has the chemical equation and rate law O 2 NNH 2 ( aq ) ⟶ N 2 O ( g ) + H 2 O ( l ) rate = k [ O 2 NNH 2 ] [ H + ] A proposed mechanism for this reaction is O 2 NNH 2 ( aq ) k 1 ⇌ k − 1 O 2 NNH − ( aq ) + H + ( aq ) ( fast equilibrium ) O 2 NNH − ( aq ) k 2 −→ N 2 O ( g ) + OH − ( aq ) ( slow ) H + ( aq ) + OH − ( aq ) k 3 −→ H 2 O ( l ) ( fast ) What is the relationship between the observed value of k and the rate constants for the individual steps of the mechanism?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
Do you know the correct answer?
The decomposition of nitramide, O 2 NNH 2 , in water has the chemical equation and rate law O 2 NNH...
Questions in other subjects:




Mathematics, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01