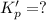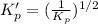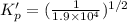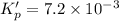Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 02:10, sativataurus
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
Do you know the correct answer?
For the following reaction, Kp = 1.9 ✕ 104 at 1722 K. H2(g) + Br2(g) equilibrium reaction arrow 2 HB...
Questions in other subjects:


Health, 30.01.2020 01:49


Biology, 30.01.2020 01:49

History, 30.01.2020 01:49

History, 30.01.2020 01:49

English, 30.01.2020 01:49


History, 30.01.2020 01:49


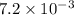
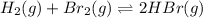 ;
; 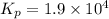
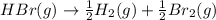 ;
;