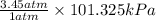Chemistry, 27.02.2020 02:55, aboatright7410
The typical pressure of carbon dioxide in an unopened soda can is 3.45 atm. Calculate the typical pressure in kPa . Be sure your answer has the right number of significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3


Chemistry, 22.06.2019 18:30, TaraC
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
Do you know the correct answer?
The typical pressure of carbon dioxide in an unopened soda can is 3.45 atm. Calculate the typical pr...
Questions in other subjects:


Mathematics, 02.07.2020 22:01

Mathematics, 02.07.2020 22:01

Mathematics, 02.07.2020 22:01

Mathematics, 02.07.2020 22:01





Mathematics, 02.07.2020 22:01