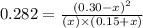Chemistry, 27.02.2020 02:01, FreyaLouise
He following reaction becomes possible: H2gI2g 2HIg The equilibrium constant K for this reaction is 0.282 at the temperature of the flask. Calculate the equilibrium molarity of H2. Round your answer to two decimal places.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, milkshakegrande101
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 05:30, choatefarmsus
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
Do you know the correct answer?
He following reaction becomes possible: H2gI2g 2HIg The equilibrium constant K for this reaction is...
Questions in other subjects:

Mathematics, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00




Mathematics, 21.05.2021 01:00



Mathematics, 21.05.2021 01:00

Chemistry, 21.05.2021 01:00


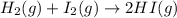
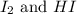
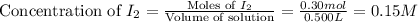
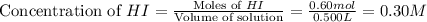
![K=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0526/1028/8a740.png)