
Consider the equilibrium reaction. 2 A + B − ⇀ ↽ − 4 C After multiplying the reaction by a factor of 2, what is the new equilibrium equation? equilibrium equation: 4A + 2B<=> 8C 4 A + 2 B − ⇀ ↽ − 8 C Create the equilibrium‑constant, K c , expression for the new equilibrium reaction.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 23.06.2019 00:30, terryg4397
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
Do you know the correct answer?
Consider the equilibrium reaction. 2 A + B − ⇀ ↽ − 4 C After multiplying the reaction by a factor of...
Questions in other subjects:






Health, 13.02.2020 20:01

Mathematics, 13.02.2020 20:01

English, 13.02.2020 20:01



![K_c=\frac{[C]^8}{[A]^4[B]^2}](/tpl/images/0525/9128/9a703.png)
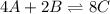
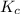 will be,
will be,



