
Chemistry, 27.02.2020 00:31, paytonrules3634
Reduction of iodate, IO3-(aq) + 5 H+(aq) + 4 e− → HIO(aq) + 2 H2O(aq), occurs with E0 = +1.13 V. What is the electrode potential, E, of the half reaction if the concentration/activity of all substances is 1.00 M?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, joclowdus99
Which is an example of renewableenergy resource uranium ,wind, or natural gas
Answers: 2

Chemistry, 21.06.2019 16:30, monithebtslover01
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 21.06.2019 17:10, codeyhatch142
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 13:10, Jana1517
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes. which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature. sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1
Do you know the correct answer?
Reduction of iodate, IO3-(aq) + 5 H+(aq) + 4 e− → HIO(aq) + 2 H2O(aq), occurs with E0 = +1.13 V. Wha...
Questions in other subjects:


Mathematics, 08.12.2021 20:40



Mathematics, 08.12.2021 20:40

Mathematics, 08.12.2021 20:40

Mathematics, 08.12.2021 20:40

Mathematics, 08.12.2021 20:40



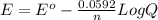
![E = E^o - \frac{0.0592}{n}LogQ\\\\E = 1.13 - \frac{0.0592}{4}Log[1]\\\\E =1.13 -0\\\\E =+1.13 V](/tpl/images/0525/9156/3694d.png)





