
Calcium nitrate will react with ammonium chloride at slightly elevated temperatures, as represented in the equation below. Ca(NO3)2(s) + 2NH4Cl(s) → 2N2O(g) + CaCl2(s) + 4H2O(g) What is the maximum volume of N2O at STP that could be produced using a 5.20-mol sample of each reactant?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, rndschopplein
Achemical reaction (also known as a chemical change) produces substances that are chemically different from the starting materials. an example of a chemical reaction is the formation of water from hydrogen and oxygen gas. in a physical change, a substance changes its physical appearance but not its chemical identity. an example of physical change is the formation of liquid water from solid water, a familiar process called melting. physically, liquid water looks very different from solid water (ice) but the chemical identity, water, is the same for both. which of following changes that affect the composition of our atmosphere involve physical changes and which involve chemical reactions? oxygen gas changes to ozone during thunderstorms carbon dioxide is produced by the combustion of gasoline in an automobile engine. when coal, oil, and natural gas are decomposed in landsills they produce methane gas. freezing rain develops when a warm air mass overrides a cold air mass. fog forms from water vapor when the temperature drops below the dew point
Answers: 1

Chemistry, 22.06.2019 21:30, rondonalba
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2

Chemistry, 23.06.2019 10:10, estebanmff
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3

Chemistry, 23.06.2019 10:30, villarrealc1987
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
Do you know the correct answer?
Calcium nitrate will react with ammonium chloride at slightly elevated temperatures, as represented...
Questions in other subjects:


Mathematics, 05.05.2020 08:07

Mathematics, 05.05.2020 08:07


Mathematics, 05.05.2020 08:07



Mathematics, 05.05.2020 08:07

Mathematics, 05.05.2020 08:07


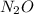 at STP produced is, 116.48 L
at STP produced is, 116.48 L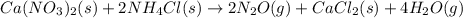
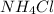 react with 1 mole of
react with 1 mole of 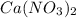
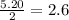 moles of
moles of 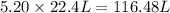 volume of gas
volume of gas 



