
Chemistry, 26.02.2020 23:32, sighgabbie
A common laboratory method for preparing a precipitate is to mix solutions containing the component ions. Does a precipitate form when 10. ml of 0.0010 M Ca(NO3)2 is mixed with 10. ml of 0.00010 M NaF? Ksp for CaF2 = 3.2 x 10-11

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, bartfrank447
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1


Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3
Do you know the correct answer?
A common laboratory method for preparing a precipitate is to mix solutions containing the component...
Questions in other subjects:



History, 03.12.2019 21:31


Mathematics, 03.12.2019 21:31


Law, 03.12.2019 21:31

Computers and Technology, 03.12.2019 21:31



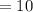 ml
ml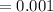
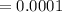

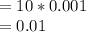
![[Ca2+] = \frac{0.01}{10 + 10} \\= \frac{0.01}{20} \\= 5 * 10^{-4}](/tpl/images/0525/7981/7884b.png)
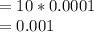
![[F-] = \frac{0.001}{10 + 10} \\= \frac{0.001}{20} \\= 5 * 10^{-5}](/tpl/images/0525/7981/81b9d.png)
![Q = [Ca2+] [F-]^2\\= (5 * 10^{-4}) * (0.5* 10^-4)\\= 1.25 * 10^{-12}](/tpl/images/0525/7981/5981e.png)





