A voltaic cell is set up with copper and hydrogen half-cells. Standard conditions are used in the copper half-cell, Cu2+ (aq, 1.00 M) | Cu (s). The hydrogen gas pressure is 1.00 bar. A value of 0.490 V is recorded for E Cell at 298 K. Determine the concentration of H+ and the pH of the solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, 23gordns
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉mo re accurate estimates can be made with the van der waals equationí‘ťí‘ť=í‘›í‘›í‘›í‘›í‘›í‘›í‘ ‰í‘‰â’푛푛푟푟â’푞푞푛푛2í‘ ‰í‘‰2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 06:30, reecedstceklein
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 21:20, paatnguyyen
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 23.06.2019 06:00, kelyanthecrafte
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
Do you know the correct answer?
A voltaic cell is set up with copper and hydrogen half-cells. Standard conditions are used in the co...
Questions in other subjects:


English, 17.04.2020 01:33

Biology, 17.04.2020 01:33




Mathematics, 17.04.2020 01:33



English, 17.04.2020 01:33


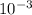 M and the pH = 2.6 of the solution
M and the pH = 2.6 of the solution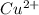 ) is the cathode and hydrogen (
) is the cathode and hydrogen (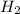 ) is the anode.
) is the anode.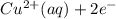 ⇒ Cu(s)
⇒ Cu(s)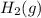 ⇒
⇒ 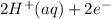
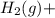
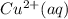 ⇒
⇒ 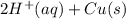

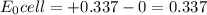
![\frac{[H^{+}]^{2} }{[Cu^{2+}]P_{H2} }](/tpl/images/0525/6315/22043.png)
 but
but 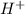 is unknown. we solve this using hernst equation.
is unknown. we solve this using hernst equation.![E = E^{0} -\frac{0.0257}{n}ln\frac{[H^{+}]^{2} }{[Cu^{2+}]P_{H2} }](/tpl/images/0525/6315/8e268.png)
![0.490 = 0.337 -\frac{0.0257}{2}ln\frac{[H^{+}]^{2} }{[1][1]}](/tpl/images/0525/6315/43505.png)
![ln{[H^{+}]^{2} } = -11.9](/tpl/images/0525/6315/8c81e.png)
![2ln{[H^{+}] } = -11.9](/tpl/images/0525/6315/31a40.png)
![ln{[H^{+}] } = -5.95](/tpl/images/0525/6315/e2ee5.png)
![[H^{+}] = 3* 10^{-3} M](/tpl/images/0525/6315/d9bb9.png)