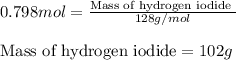Chemistry, 26.02.2020 22:32, crzyemo865
For the chemical reaction below, determine the amount of HI produced when 3.32E+0 g of hydrogen is reacted with 5.064E+1 g of iodine to produce hydrogen iodide (HI). H(g) + I(g) → 2HI(g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, lilque6112
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
Do you know the correct answer?
For the chemical reaction below, determine the amount of HI produced when 3.32E+0 g of hydrogen is r...
Questions in other subjects:


Mathematics, 08.01.2021 20:40

Geography, 08.01.2021 20:40

Mathematics, 08.01.2021 20:40

French, 08.01.2021 20:40

Mathematics, 08.01.2021 20:40

Advanced Placement (AP), 08.01.2021 20:40

Mathematics, 08.01.2021 20:40


History, 08.01.2021 20:40


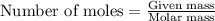 .....(1)
.....(1)
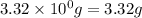
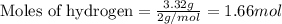
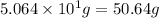
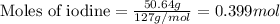
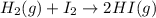
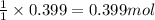 of hydrogen
of hydrogen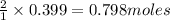 of hydrogen iodide
of hydrogen iodide