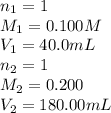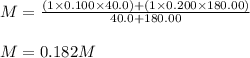Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1


Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
Do you know the correct answer?
A solution was prepared by mixing 40.00 mL of 0.100 M HNO3 and 180.00 mL of 0.200 M HNO3 . Calculate...
Questions in other subjects:

Arts, 23.04.2020 00:50






Biology, 23.04.2020 00:51


Biology, 23.04.2020 00:51

Mathematics, 23.04.2020 00:51


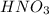 in the final solution is 0.182 M
in the final solution is 0.182 M
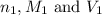 are the n-factor, molarity and volume of the
are the n-factor, molarity and volume of the 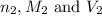 are the n-factor, molarity and volume of the
are the n-factor, molarity and volume of the