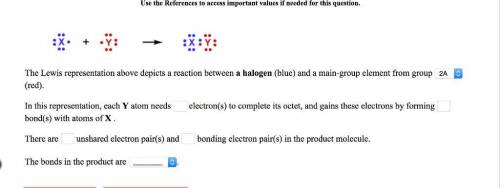
The lewis representation above depicts a reaction between a halogen (blue) and a main-group element from group (red). in this representation, each y atom needs electron(s) to complete its octet, and gains these electrons by forming bond(s) with atoms of x . there are unshared electron pair(s) and bonding electron pair(s) in the product molecule. the bonds in the product are

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
Do you know the correct answer?
The lewis representation above depicts a reaction between a halogen (blue) and a main-group element...
Questions in other subjects:


History, 25.03.2021 19:30

Mathematics, 25.03.2021 19:30

Computers and Technology, 25.03.2021 19:30

Mathematics, 25.03.2021 19:30


Mathematics, 25.03.2021 19:30

Mathematics, 25.03.2021 19:30