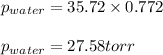Chemistry, 26.02.2020 20:52, cocomorillo35181
The ambient temperature is 85.0°F and the humidity of the surrounding air is reported to be 68.0%. Using the Clausius-Clapeyron equation and the boiling point of water as 100.0°C at 760 torr, calculate the vapor pressure (in torr) of water in the air. Use 40.7 kJ/mol as the ∆Hvap of water.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3


Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
Do you know the correct answer?
The ambient temperature is 85.0°F and the humidity of the surrounding air is reported to be 68.0%. U...
Questions in other subjects:

Geography, 13.03.2021 03:30


Physics, 13.03.2021 03:30



English, 13.03.2021 03:30


English, 13.03.2021 03:30

Mathematics, 13.03.2021 03:30

Spanish, 13.03.2021 03:30


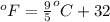
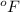 = temperature in Fahrenheit
= temperature in Fahrenheit 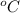 = temperature in centigrade
= temperature in centigrade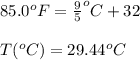
![\ln(\frac{P_2}{P_1})=\frac{\Delta H}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0525/3984/5c76e.png)
 = initial pressure which is the pressure at normal boiling point = 760 torr
= initial pressure which is the pressure at normal boiling point = 760 torr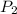 = final pressure = ?
= final pressure = ?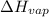 = Enthalpy of vaporization = 40.7 kJ/mol = 40700 J/mol (Conversion factor: 1 kJ = 1000 J)
= Enthalpy of vaporization = 40.7 kJ/mol = 40700 J/mol (Conversion factor: 1 kJ = 1000 J)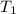 = initial temperature =
= initial temperature = ![100^oC=[100+273]K=373K](/tpl/images/0525/3984/44e24.png)
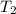 = final temperature =
= final temperature = ![29.44^oC=[29.44+273]=302.44K](/tpl/images/0525/3984/ddd83.png)
![\ln(\frac{P_2}{760})=\frac{40700J/mol}{8.314J/mol.K}[\frac{1}{373}-\frac{1}{302.44}]\\\\P_2=35.72torr](/tpl/images/0525/3984/e0926.png)
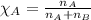

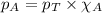
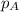 = vapor pressure of water = ?
= vapor pressure of water = ?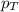 = total pressure = 35.72 torr
= total pressure = 35.72 torr = mole fraction of water = 0.774
= mole fraction of water = 0.774