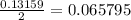Chemistry, 26.02.2020 19:29, whitneyt3218
For the reaction Ti(s)+2F2(g)→TiF4(s) compute the theoretical yield of the product (in grams) for each of the following initial amounts of reactants. Part A 5.0 g Ti, 5.0 g F2 Express your answer using two significant figures.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, miamassimino
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3
Do you know the correct answer?
For the reaction Ti(s)+2F2(g)→TiF4(s) compute the theoretical yield of the product (in grams) for ea...
Questions in other subjects:

Biology, 19.10.2021 14:00


Health, 19.10.2021 14:00


English, 19.10.2021 14:00



Biology, 19.10.2021 14:00


Mathematics, 19.10.2021 14:00


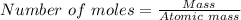
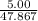
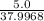
 mole of Ti will react with the 0.13159 mole of F₂
mole of Ti will react with the 0.13159 mole of F₂