
Chemistry, 26.02.2020 17:55, shadowselena63
The rate constant of a reaction is 6.85 × 10−5 L/mol·s at 195°C and 2.20 × 10−3 L/mol·s at 258°C. What is the activation energy of the reaction? Enter your answer in scientific notation.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1


Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
Do you know the correct answer?
The rate constant of a reaction is 6.85 × 10−5 L/mol·s at 195°C and 2.20 × 10−3 L/mol·s at 258°C. Wh...
Questions in other subjects:

Mathematics, 17.10.2020 01:01

Mathematics, 17.10.2020 01:01




English, 17.10.2020 01:01





![\ln(\frac{K_{258^oC}}{K_{195^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0525/0415/d01a9.png)
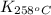 = equilibrium constant at 258°C =
= equilibrium constant at 258°C = 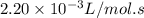
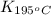 = equilibrium constant at 195°C =
= equilibrium constant at 195°C = 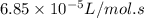
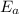 = Activation energy of the reaction = ?
= Activation energy of the reaction = ?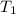 = initial temperature =
= initial temperature = ![195^oC=[195+273]K=468K](/tpl/images/0525/0415/d4d73.png)
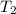 = final temperature =
= final temperature = ![258^oC=[258+273]K=531K](/tpl/images/0525/0415/697bf.png)
![\ln(\frac{2.20\times 10^{-3}}{6.85\times 10^{-5}})=\frac{E_a}{8.314J/mol.K}[\frac{1}{468}-\frac{1}{531}]\\\\E_a=113780J/mol=113.8kJ/mol](/tpl/images/0525/0415/e4c71.png)




