
Chemistry, 26.02.2020 17:47, clairebear66
Given the following balanced equation, determine the rate of reaction with respect to [O2]. If the rate of formation of O2 is 7.78 x 10-1 M/s, what is the rate of the loss of O3? 2 O3(g) → 3 O2(g)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1
Do you know the correct answer?
Given the following balanced equation, determine the rate of reaction with respect to [O2]. If the r...
Questions in other subjects:


Computers and Technology, 22.05.2020 19:57

Biology, 22.05.2020 19:57




History, 22.05.2020 19:57




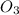 is 0.52M/s
is 0.52M/s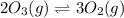
![-\frac{1d[O_3]}{2dt}](/tpl/images/0525/0164/0b459.png)
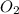 =
=![+\frac{1d[O_2]}{3dt}](/tpl/images/0525/0164/4dcb2.png)
![-\frac{1d[O_3]}{2dt}=+\frac{1d[O_2]}{3dt}](/tpl/images/0525/0164/b3aa3.png)
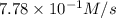
![\frac{2d[O_2]}{3dt}=\frac{2}{3}\times 7.78\times 10^{-1}M/s=0.52M/s](/tpl/images/0525/0164/964d0.png)




