
Chemistry, 26.02.2020 17:01, vanessasantos2004vs
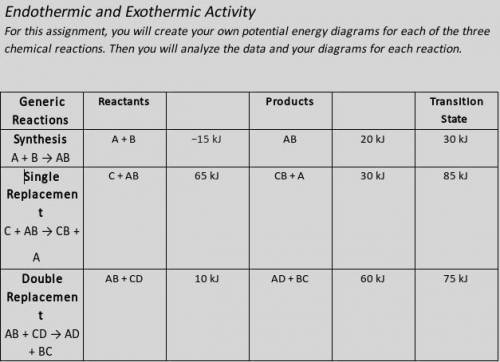
Illustrate the x- and y-axes to show the reaction pathway and potential energy, in kilojoules. Ensure your energy intervals are appropriate for the data.
Plot the enthalpy values of the reactants, products, and transition state using three horizontal dotted lines across the graph for each.
Draw the energy curve from the reactants line to the transition state and curve the line back down to the energy of the products. Label the reactants, products, and transition state.
Illustrate double-headed arrows to represent both the total change in enthalpy (ΔH) and the activation energy (Ea).
Calculate the total change in enthalpy and the activation energy using the energy values provided for each reaction. Record those values below the graph.
Make sure correct units are included.
I know this is a lot, so I'm giving more points than I ever have. Thank you!!!

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1


Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
Do you know the correct answer?
Illustrate the x- and y-axes to show the reaction pathway and potential energy, in kilojoules. Ensur...
Questions in other subjects:


Mathematics, 09.03.2021 14:00



Mathematics, 09.03.2021 14:00



Mathematics, 09.03.2021 14:00

English, 09.03.2021 14:00

Mathematics, 09.03.2021 14:00






