

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, leannamat2106
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1


Chemistry, 23.06.2019 10:30, fatheadd2007
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

Chemistry, 23.06.2019 13:00, rachael382
How does the kinetic energy of a substance's particle in the solid phase compare to their kinetic enegy in the liquid phase?
Answers: 1
Do you know the correct answer?
A concentration cell consists of two Sn/Sn2+ half-cells. The cell has a potential of 0.10 V at 25 ºC...
Questions in other subjects:



Mathematics, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00


Mathematics, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00

English, 19.01.2021 14:00


![\frac{[Sn^{2+}_{(diluted)}}{[Sn^{2+}_{(concentrated)}]}](/tpl/images/0524/9528/81ffa.png) in the cell is
in the cell is 

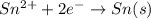
 will be equal to zero.
will be equal to zero.![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Sn^{2+}_{(diluted)}}{[Sn^{2+}_{(concentrated)}]}](/tpl/images/0524/9528/cb590.png)
 = 0.10 V
= 0.10 V![\frac{[Sn^{2+}_{(diluted)}}{[Sn^{2+}_{(concentrated)}]}](/tpl/images/0524/9528/5c0ba.png) = ?
= ?![0.10=0-\frac{0.0592}{2}\log \frac{[Sn^{2+}_{(diluted)}}{[Sn^{2+}_{(concentrated)}]}](/tpl/images/0524/9528/25d4c.png)
![\frac{[Sn^{2+}_{(diluted)}}{[Sn^{2+}_{(concentrated)}]}=4.016\times 10^{-4}](/tpl/images/0524/9528/6ddca.png)





