Chemistry, 26.02.2020 16:11, lanaasad7292
Nitric oxide is made from the oxidation of ammonia. What mass of nitric oxide can be made from the reaction of 8.00 g NH 3 with 17.0 g O 2? 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
Do you know the correct answer?
Nitric oxide is made from the oxidation of ammonia. What mass of nitric oxide can be made from the r...
Questions in other subjects:


History, 03.08.2019 03:00

Health, 03.08.2019 03:00


Health, 03.08.2019 03:00

Biology, 03.08.2019 03:00


English, 03.08.2019 03:00


Mathematics, 03.08.2019 03:00


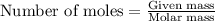 .....(1)
.....(1)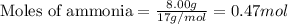
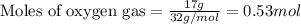
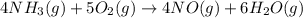
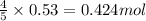 of ammonia
of ammonia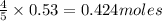 of NO
of NO