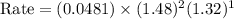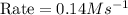For the reaction, A(g) + B(g) => AB(g), the rate is 0.385 mol/L. s when the initial concentrations of both A and B are 2.00 mol/L. If the reaction is second order in A and first order in B, what is the rate when the initial concentration of A = 1.48 mol/L and that of B = 1.32 mol/L. Give your answer to 2 decimal places

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
Do you know the correct answer?
For the reaction, A(g) + B(g) => AB(g), the rate is 0.385 mol/L. s when the initial concentration...
Questions in other subjects:











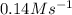
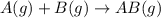
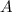 and
and 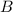 are the reactants.
are the reactants.![\text{Rate}=k[A]^2[B]^1](/tpl/images/0524/7200/858f4.png)
![\text{Rate}=k[A]^2[B]](/tpl/images/0524/7200/15407.png)
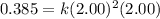
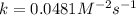
![\text{Rate}=k[A]^2[B]^0[C]^1](/tpl/images/0524/7200/54afd.png)