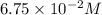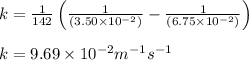Chemistry, 26.02.2020 05:43, Jadaflournoy5
Hydrogen iodide decomposes at 800 K via a second-order process to produce hydrogen and iodine according to the following chemical equation. 2 HI(g) ---> H2 (g) + I2 g) At 800 K it takes 142 seconds for the initial concentration of HI to decrease from 6.75 x 10^-2 M to 3.50 x 10^-2 M. What is the rate constant for the reaction at this temperature?
A) 5.12 x 10^-4 M^-1 s^-1
B) 9.69 x 10^-2 M^-1 s^-1
C) 10.3 M^-1 s^-1
D) 1.95 x 10^3 M^-1 s^-1

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Do you know the correct answer?
Hydrogen iodide decomposes at 800 K via a second-order process to produce hydrogen and iodine accord...
Questions in other subjects:

Biology, 10.11.2020 21:40

Mathematics, 10.11.2020 21:40

Chemistry, 10.11.2020 21:40

History, 10.11.2020 21:40

Mathematics, 10.11.2020 21:40

Computers and Technology, 10.11.2020 21:40

English, 10.11.2020 21:40



Geography, 10.11.2020 21:40


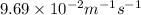
![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0524/6600/5ea71.png)
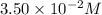
![[A]_o](/tpl/images/0524/6600/9caf5.png) = Initial concentration =
= Initial concentration =