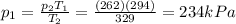On a summer day, you take a road trip through Death Valley, California, in an antique car. You start out at a temperature of 21°C, but the temperature in Death Valley will reach a peak of 56°C. The tires on your car hold 13.2 L of nitrogen gas at a starting pressure of 240 kPa. The tires will burst when the internal pressure (Pb) reaches 262 kPa. Answer the following questions and show your work.
• How many moles of nitrogen gas are in each tire?
• What will the tire pressure be at peak temperature in Death Valley?
• Will the tires burst in Death Valley? Explain.
• If you must let nitrogen gas out of the tire before you go, to what pressure must you reduce the tires before you start your trip? (Assume no significant change in tire volume.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3


Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1
Do you know the correct answer?
On a summer day, you take a road trip through Death Valley, California, in an antique car. You start...
Questions in other subjects:




Mathematics, 29.09.2019 15:10


History, 29.09.2019 15:10





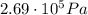
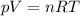
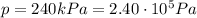
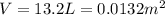
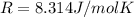
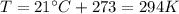
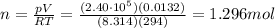
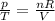
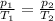
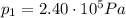 is the initial pressure
is the initial pressure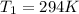 is the initial temperature
is the initial temperature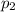 is the final pressure in Death Valley
is the final pressure in Death Valley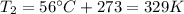 is the temperature in Death Valley
is the temperature in Death Valley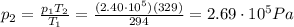
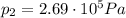
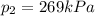
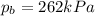
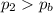
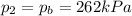
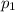 , the initial pressure at which the tires should be in order not to burst when the car arrives in the Death Valley.
, the initial pressure at which the tires should be in order not to burst when the car arrives in the Death Valley.