
Chemistry, 26.02.2020 02:23, jcrowley9362
Calcium carbide, CaC2, used to produce acetylene, C2H2, is prepared by heating calcium oxide and carbon to high temperature. Carbon monoxide gas is formed as a by-product. a. Write down the balanced equation for this process. b. If a mixture contains 1.15 kg of each reactant, how many grams of calcium carbide can be prepared?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, EMQPWE
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1
Do you know the correct answer?
Calcium carbide, CaC2, used to produce acetylene, C2H2, is prepared by heating calcium oxide and car...
Questions in other subjects:

Mathematics, 23.10.2020 06:01




Mathematics, 23.10.2020 06:01

Mathematics, 23.10.2020 06:01

Mathematics, 23.10.2020 06:01


Computers and Technology, 23.10.2020 06:01


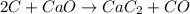
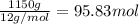
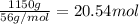
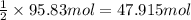 of CaO.
of CaO.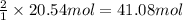 of carbon
of carbon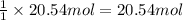 of calcium carbide
of calcium carbide



