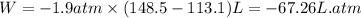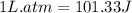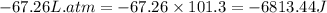Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, nique0808
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 23.06.2019 01:50, kayleebueno
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
Do you know the correct answer?
The combustion of 0.25 moles of octane gas (C8H18) to CO2 gas and H2O gas (vapor) against a constant...
Questions in other subjects:











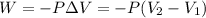
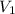 = initial volume = 113.1 L
= initial volume = 113.1 L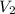 = final volume = 148.5 L
= final volume = 148.5 L