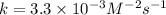Chemistry, 26.02.2020 00:55, 02s54mcoupe
For the reaction A +B+ C D E, the initial reaction rate was measured for various initial concentrations of reactants. The following data were collected Trial A Bl Cl Initial rate 0.30 0.30 0.30 9.0x10 o 2.7x10-4 0.30 0.30 0.90 3.6x10-4 0.60 0.30 0.30 3.6x10-4 0.60 0.60 0.30
What is the value of the rate constant k for this reaction? When entering compound units, indicate multiplication of units explicitly using a multiplication dot (multiplication dot in the menu). For example, M−1⋅s−1. Express your answer to two significant figures and include the appropriate units. Indicate the multiplication of units explicitly either with a multiplication dot or a dash.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
Do you know the correct answer?
For the reaction A +B+ C D E, the initial reaction rate was measured for various initial concentrati...
Questions in other subjects:

Health, 24.06.2019 23:30




Health, 24.06.2019 23:30





History, 24.06.2019 23:30


 .
.![R=k[A]^a[B]^b[C]^c](/tpl/images/0524/1142/87958.png)
![[A]=0.30 M,[B]=0.30 M,[C]=0.30 M](/tpl/images/0524/1142/51008.png)
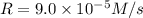
![9.0\times 10^{-5} M/s=k[0.30 M]^a[0.30 M]^b[0.30 M]^c](/tpl/images/0524/1142/34466.png) ...[1]
...[1]![[A]=0.30 M,[B]=0.30 M,[C]=0.90 M](/tpl/images/0524/1142/e516b.png)
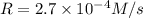
![2.7\times 10^{-4} M/s=k[0.30 M]^a[0.30 M]^b[0.90 M]^c](/tpl/images/0524/1142/a5767.png) ...[2]
...[2]![[A]=0.60 M,[B]=0.30 M,[C]=0.30 M](/tpl/images/0524/1142/90b57.png)
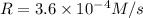
![3.6\times 10^{-4} M/s=k[0.60 M]^a[0.30 M]^b[0.30 M]^c](/tpl/images/0524/1142/97780.png) ...[3]
...[3]![[A]=0.60 M,[B]=0.60 M,[C]=0.30 M](/tpl/images/0524/1142/aaf09.png)
![3.6\times 10^{-4} M/s=k[0.60 M]^a[0.60 M]^b[0.30 M]^c](/tpl/images/0524/1142/d2528.png) ...[4]
...[4]![R=k[A]^2[B]^0[C]^1](/tpl/images/0524/1142/487c6.png)
![9.0\times 10^{-5} M/s=k[0.30 M]^2[0.30 M]^0[0.30 M]^1](/tpl/images/0524/1142/e707a.png)
![k=\frac{9.0\times 10^{-5} M/s}{[0.30 M]^2[0.30 M]^0[0.30 M]^1}](/tpl/images/0524/1142/db6d7.png)