
Consider the following elementary reaction:
N2O—> N2 + O
suppose we let K1 stand for the rate constant of this reaction, and k-1 stand for the rate constant of the reverse reaction. write an expression that gives the equilibrium concentration of N2O in terms of k1, k-1 and the equilibrium concentrations of N2 and O

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:00, brittanygibson2812
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 00:10, bossboybaker
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2
Do you know the correct answer?
Consider the following elementary reaction:
N2O—> N2 + O
suppose we let K1 stand fo...
N2O—> N2 + O
suppose we let K1 stand fo...
Questions in other subjects:



English, 03.01.2020 22:31

English, 03.01.2020 22:31

English, 03.01.2020 22:31

Social Studies, 03.01.2020 22:31

Mathematics, 03.01.2020 22:31


Health, 03.01.2020 22:31


![[N_2O]=\frac{K^{-1}}{K_1}\times [N_2][O_2]](/tpl/images/0524/0986/7a4ba.png)
![R_f=K_1\times [N_2O]](/tpl/images/0524/0986/5d806.png)
![R_b=K^{-1}\times [N_2][O_2]](/tpl/images/0524/0986/327e3.png)
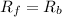
![K_1\times [N_2O]=K^{-1}\times [N_2][O_2]](/tpl/images/0524/0986/21d13.png)




