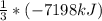Chemistry, 25.02.2020 22:49, alicciardone01
The enthalpy change for the explosion of ammonium nitrate with fuel oil is –7198 kJ for every 3 moles of NH4NO3. What is the enthalpy change for 1.0 mole of NH4NO3 in this reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3


Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
Do you know the correct answer?
The enthalpy change for the explosion of ammonium nitrate with fuel oil is –7198 kJ for every 3 mole...
Questions in other subjects:

Biology, 27.09.2020 07:01


History, 27.09.2020 07:01

Spanish, 27.09.2020 07:01



English, 27.09.2020 07:01



Biology, 27.09.2020 07:01


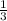 of the total enthaply of the 3 moles
of the total enthaply of the 3 moles