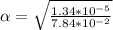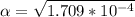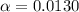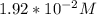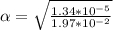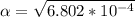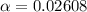Chemistry, 25.02.2020 22:51, tmmackie1748
Calculate the percent ionization of propionic acid (C2H5COOH) in solutions of each of the following concentrations (Ka is given in Appendix D in the textbook).
Part A. 0.270 M
Part B. 7.84×10-2 M
Part C. 1.97×10-2 M

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 22:30, needhelpasap8957
Why is the bottom layer of a trophic pyrimid the
Answers: 2

Chemistry, 23.06.2019 02:00, FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
Do you know the correct answer?
Calculate the percent ionization of propionic acid (C2H5COOH) in solutions of each of the following...
Questions in other subjects:



English, 26.07.2021 17:50






Biology, 26.07.2021 17:50

Mathematics, 26.07.2021 17:50


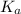 =
= 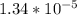
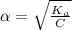
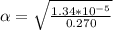
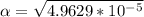
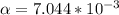
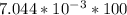
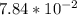 M
M