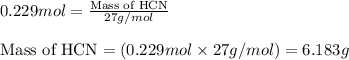Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process: Hydrogen cyanide is used to prepare sodium cyanide, which is used in part to obtain gold from gold-containing rock. If a reaction vessel contains 5.90 g NH3, 11.0 g O2, and 4.67 g CH4, what is the maximum mass in grams of hydrogen cyanide that could be made, assuming the reaction goes to completion as written?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 03:30, vaehcollier
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
Do you know the correct answer?
Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas (CH4) by the following process...
Questions in other subjects:


Health, 01.09.2019 05:30

Social Studies, 01.09.2019 05:30

Geography, 01.09.2019 05:30



Social Studies, 01.09.2019 05:30

History, 01.09.2019 05:30

English, 01.09.2019 05:30

Social Studies, 01.09.2019 05:30


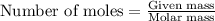 .....(1)
.....(1)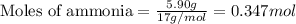
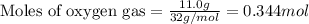
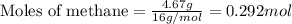
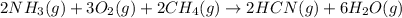
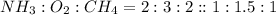
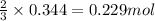 of HCN
of HCN