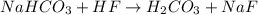Chemistry, 25.02.2020 22:06, AmyGonzalez1385
1. Acidification of sodium bicarbonate (NaHCO3) produces carbon dioxide in a two-step process. The first step produces carbonic acid (H2CO3). Write and balance the molecular equation for the first step of the reaction of sodium bicarbonate with HF.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:50, ddmoorehouseov75lc
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3


Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1
Do you know the correct answer?
1. Acidification of sodium bicarbonate (NaHCO3) produces carbon dioxide in a two-step process. The f...
Questions in other subjects:






Biology, 15.06.2021 21:10