
Chemistry, 25.02.2020 21:45, Kelshonti15
For the reaction, A(g) + B(g) => AB(g), the rate is 0.625 mol/L. s when the initial concentrations of both A and B are 2.00 mol/L. If the reaction is first order in A and first order in B, what is the rate when the initial concentration of A = 4.41 mol/L and that of B = 2.72 mol/L. Give answer to 2 decimal places.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1
Do you know the correct answer?
For the reaction, A(g) + B(g) => AB(g), the rate is 0.625 mol/L. s when the initial concentration...
Questions in other subjects:


Mathematics, 14.12.2019 09:31



English, 14.12.2019 09:31

Arts, 14.12.2019 09:31


English, 14.12.2019 09:31

Health, 14.12.2019 09:31

Chemistry, 14.12.2019 09:31


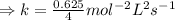
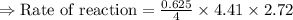
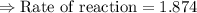 ≈1.87 mol L⁻¹s⁻¹
≈1.87 mol L⁻¹s⁻¹



