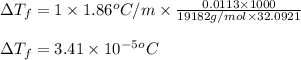A biochemical engineer isolates a bacterial gene fragment and dissolves an 11.3 mg sample of the material in enough water to make 32.2 mL of solution. The osmotic pressure of the solution is 0.340 torr at 25°C.
(a) What is the molar mass of the gene fragment?
(b) If the solution density is 0.997 g/mL, how large is the freezing point depression for this solution (Kf of water = 1.86 °C/m)?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, johnnydenali67
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
Do you know the correct answer?
A biochemical engineer isolates a bacterial gene fragment and dissolves an 11.3 mg sample of the mat...
Questions in other subjects:

Mathematics, 19.01.2021 20:20

History, 19.01.2021 20:20



English, 19.01.2021 20:20


Mathematics, 19.01.2021 20:20



Mathematics, 19.01.2021 20:20


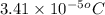
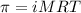
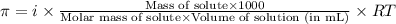
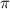 = osmotic pressure of the solution = 0.340 torr
= osmotic pressure of the solution = 0.340 torr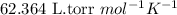
![25^oC=[273+25]=298K](/tpl/images/0523/6100/6a9f9.png)

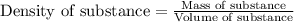
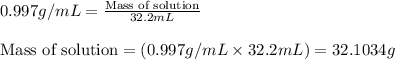
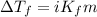
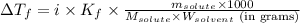
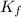 = molal freezing point elevation constant = 1.86°C/m
= molal freezing point elevation constant = 1.86°C/m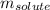 = Given mass of solute (gene fragment) = 0.0113 g
= Given mass of solute (gene fragment) = 0.0113 g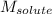 = Molar mass of solute (gene fragment) = 19182 g/mol
= Molar mass of solute (gene fragment) = 19182 g/mol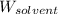 = Mass of solvent (water) = 32.0921 g
= Mass of solvent (water) = 32.0921 g