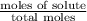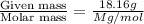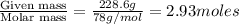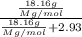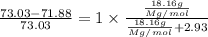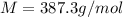Chemistry, 25.02.2020 19:59, rhineharttori
The common laboratory solvent benzene is often used to purify substances dissolved in it. The vapor pressure of benzene , C6H6, is 73.03 mm Hg at 25 degrees Celsius.
In a laboratory experiment, students synthesized a new compound and found that when 18.16 grams of the compound were dissolved in 228.6 grams of benzene, the vapor pressure of the solution was 71.88 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte.
What is the molecular weight of this compound ?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1


Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
Do you know the correct answer?
The common laboratory solvent benzene is often used to purify substances dissolved in it. The vapor...
Questions in other subjects:



Biology, 08.12.2020 06:30

Mathematics, 08.12.2020 06:30

Chemistry, 08.12.2020 06:30

Mathematics, 08.12.2020 06:30



Mathematics, 08.12.2020 06:30

Mathematics, 08.12.2020 06:30


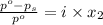
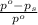 = relative lowering in vapor pressure
= relative lowering in vapor pressure
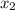 = mole fraction of solute =
= mole fraction of solute =