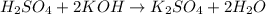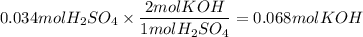The reaction of sulfuric acid (H2SO4) with potassium hydroxide (KOH) is described by the equation below. Suppose
0.06 L of KOH with unknown concentration is placed in a flask with bromthymol blue indicator. A solution of 0.20 M
H2504 is dripped into the KOH solution. After exactly 0.017 L of H2SO4 is added, the Indicator changes from blue to
yellow. What is the concentration of the KOH? You must show all of your work to earn credit. (4 points)
H2SO4 + 2KOH → K2SO4 + 2H20

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 04:10, NavyCo
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1

Chemistry, 23.06.2019 19:30, miguelturner
The total amount of fresh water on earth is estimated to be 3.73 x 10^8 km^3. what is this volume in cubic meters? in cubic feet?
Answers: 1

Chemistry, 23.06.2019 21:00, marivi3cazares
Examine these two msds from different manufacturers for sodium hydroxide (naoh). compare and contrast the following aspects: chemical names, chemical properties, order of components, health hazards, and proper disposal. click on each of the links to examine two msds reports from different sources. sodium hydroxide version 1 sodium hydroxide version 2
Answers: 1
Do you know the correct answer?
The reaction of sulfuric acid (H2SO4) with potassium hydroxide (KOH) is described by the equation be...
Questions in other subjects:


Health, 02.12.2021 05:10

Social Studies, 02.12.2021 05:10

Mathematics, 02.12.2021 05:10




Physics, 02.12.2021 05:10

Mathematics, 02.12.2021 05:10