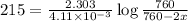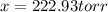Dinitrogen pentoxide decomposes in the gas phase to form nitrogen dioxide and oxygen gas. The reaction is first order in dinitrogen pentoxide and has a half-life of 2.81 h at 25 ∘C. If a 1.7 −L reaction vessel initially contains 755 torr of N2O5 at 25 ∘C, what partial pressure of O2 will be present in the vessel after 215 minutes?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
Do you know the correct answer?
Dinitrogen pentoxide decomposes in the gas phase to form nitrogen dioxide and oxygen gas. The reacti...
Questions in other subjects:

Mathematics, 28.08.2019 22:00


Chemistry, 28.08.2019 22:00

Geography, 28.08.2019 22:00

Social Studies, 28.08.2019 22:00



History, 28.08.2019 22:00

Chemistry, 28.08.2019 22:00


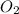 is, 222.93 torr
is, 222.93 torr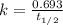
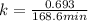
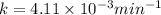
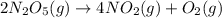
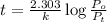
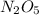 = 760 torr
= 760 torr