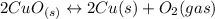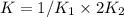Chemistry, 25.02.2020 06:14, andrewgainey1986
Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g) <--> 2Cu2O(s), K1 2CuO(s) <--> Cu2O(s) + 1/2 O2(g), K2 what is K for the system 2Cu(s) + O2(g) <--> 2CuO(s) equivalent to?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
Do you know the correct answer?
Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g) <--> 2Cu2O(s), K1...
Questions in other subjects:




Mathematics, 19.05.2020 13:01

Mathematics, 19.05.2020 13:01



Mathematics, 19.05.2020 13:01


English, 19.05.2020 13:01


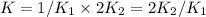
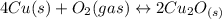
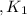 assuming equation (1)
assuming equation (1)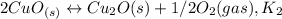 assuming equation (2)
assuming equation (2)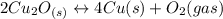 ,
,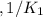 assuming equation (3)
assuming equation (3)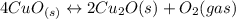
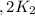 assuming equation (4)
assuming equation (4)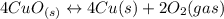
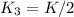 , equation (5)
, equation (5)