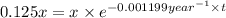Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1
Do you know the correct answer?
A decomposition reaction has a half life of 578 years. (a) What is the rate constant for this reacti...
Questions in other subjects:






History, 03.10.2019 07:00

Physics, 03.10.2019 07:00


Mathematics, 03.10.2019 07:00

Mathematics, 03.10.2019 07:00


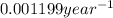 is the rate constant for this reaction.
is the rate constant for this reaction.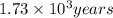 to concentration to reach 12.5% of its original value.
to concentration to reach 12.5% of its original value.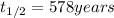
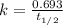
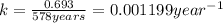
![[A_o]](/tpl/images/0522/9110/dc622.png) = x
= x![[A]](/tpl/images/0522/9110/6aa06.png) = 12.5% of x = 0.125x
= 12.5% of x = 0.125x![[A]=[A_o]\times e^{-kt}](/tpl/images/0522/9110/abdec.png)