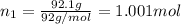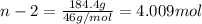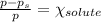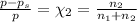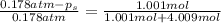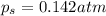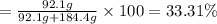Chemistry, 25.02.2020 05:17, paytonxxburns05
An ideal solution containing 92.1 g of glycerin, C3H5(OH)3, and 184.4 g of ethanol, C2H5OH, is at 40°C. The vapor pressure of pure ethanol is 0.178 atm at 40°C. Glycerin is essentially nonvolatile at this temperature. Compute the vapor pressure and weight percentage of Glycerin.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
Do you know the correct answer?
An ideal solution containing 92.1 g of glycerin, C3H5(OH)3, and 184.4 g of ethanol, C2H5OH, is at 40...
Questions in other subjects:

Mathematics, 04.08.2019 16:30


Biology, 04.08.2019 16:30

Physics, 04.08.2019 16:30






Biology, 04.08.2019 16:30